We study how information is processed in neuronal networks to drive behavior
Our interests
- How do neurons sense environmental cues, such as odorants and tastants?
- How do neurons integrate information from different cues or different neurons?
- How do neurons interact to make functional modules?
- How do neurons drive behavior and how may the behavioral state affect neuronal activity?
- How are neurons modulated by context and experience?
- How do neurons store, retrieve and forget information?
Our lab studies molecular, physiological and computational aspects of these questions, and we study them in different scales, from single neurons to neuronal networks.
Our model organism
One of the main challenges in studying neuronal networks is the huge complexity and scale of the brain, with ~1011 neurons. In contrast, the nematode C. elegans has only 302 neurons and known connectivity. C. elegans responds to a variety of environmental cues and generates a multitude of behavioral responses that are modulated by context and experience. Overall, the C. elegans nervous system is compact yet generates complex functions, rendering it a great model for studying how neuronal networks process information to drive behavior.
Our approach
- We study how neuronal features (e.g. activity, gene expression) and animal behavior are modulated by environmental cues, neuronal perturbations, genetic perturbations, and behavioral states.
- To faithfully study how the neuronal network functions, we design systematic experiments to explore responses to a wide range of environmental cues, and functions of many neurons or genes. We develop high-throughput technologies to support this approach.
Our techniques
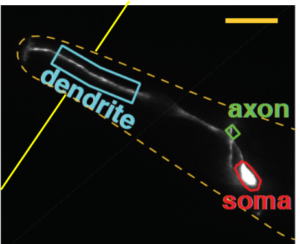 We use microfluidic devices to image neuronal activity in response to controlled environmental cues. Here the worm is immobilized with its head poking into a flow channel. Neuronal activity (GCaMP) is measured in response to various stimuli presented in the flow channel.
We use microfluidic devices to image neuronal activity in response to controlled environmental cues. Here the worm is immobilized with its head poking into a flow channel. Neuronal activity (GCaMP) is measured in response to various stimuli presented in the flow channel.
 We develop high-throughput microfluidic setups to analyze animal behavior in response to controlled environmental cues. Here, a variety of behaviors are analyzed in response to a controlled odor gradient. Our setups simultaneously and automatically monitor hundreds of free-moving animals. This allows systematic behavior analysis in a wide range of environmental conditions, and systematic evaluation of functions of many neurons (strains with perturbed neurons) or genes (strains with genetic perturbations).
We develop high-throughput microfluidic setups to analyze animal behavior in response to controlled environmental cues. Here, a variety of behaviors are analyzed in response to a controlled odor gradient. Our setups simultaneously and automatically monitor hundreds of free-moving animals. This allows systematic behavior analysis in a wide range of environmental conditions, and systematic evaluation of functions of many neurons (strains with perturbed neurons) or genes (strains with genetic perturbations).
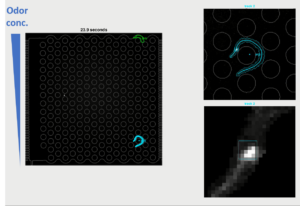 We develop microfluidic setups to analyze behavior and neuronal activity while animals are freely navigating in controlled environments. Here, we monitor the AWCON sensory neuron as the animals navigate in a controlled odor (butanone) gradient. Regularly, this sensory neuron is activated when butanone levels are decreased. However, we found that when the animal is moving down the odor gradient, initiation of neuronal activity is delayed (e.g in this movie). Why is sensation delayed and what set the delay time? In which conditions will the sensory neuron be activated, and what is the underlying neuronal computation? What is the molecular basis of this delay? Why delaying – what may be the benefit of delayed sensory responses? You can find some of the answers here.
We develop microfluidic setups to analyze behavior and neuronal activity while animals are freely navigating in controlled environments. Here, we monitor the AWCON sensory neuron as the animals navigate in a controlled odor (butanone) gradient. Regularly, this sensory neuron is activated when butanone levels are decreased. However, we found that when the animal is moving down the odor gradient, initiation of neuronal activity is delayed (e.g in this movie). Why is sensation delayed and what set the delay time? In which conditions will the sensory neuron be activated, and what is the underlying neuronal computation? What is the molecular basis of this delay? Why delaying – what may be the benefit of delayed sensory responses? You can find some of the answers here.
We also combine our microfluidics and imaging technologies with other experimental techniques, including: optogenetics and chemogenetics for acute activation or silencing of neurons, various genetic techniques for targeting specific neurons or genes, whole brain imaging for simultaneous monitoring of all neurons in the worm head, and gene expression profiling.
To understand what neuronal networks compute, we combine these quantitative experimental approaches with mathematical modeling. See example here.
